September is Thyroid Cancer Awareness Month. At GoPath, we strive to create diagnostics that are in the best interests of all cancer patients. During Thyroid Cancer Awareness Month, we would like to introduce one of our most recent developments, ThyroiNowTM. This highly sensitive and specific test not only can diagnose thyroid cancer, but it can be especially useful for differentiating "indeterminate" and suspicious thyroid FNA biopsies.
Why ThyroiNowTM?
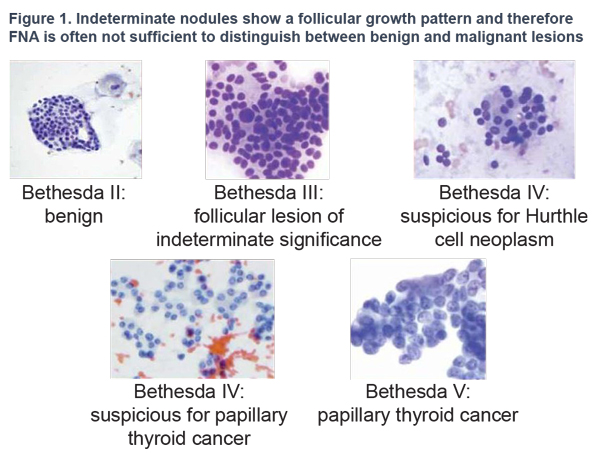
Thyroid cancer typically presents itself as a nodule without clinical symptoms. Differential diagnosis currently relies on Bethesda criteria combined with cytology findings from FNA biopsies. However, one-third of nodules are classified as "indeterminate" by a cytopathologist and only 10-40% prove to be malignant after surgery.1 Considering the high cost and possible complications caused by surgery, a more accurate and definitive diagnostic test was needed.
The ThyroiNowTM Advantage
Molecular testing for common somatic mutations has been shown to be useful for detecting thyroid cancer in "indeterminate" and "suspicious" thyroid FNA biopsies. To do this, we use cutting-edge, droplet digital PCR technology that allows us to detect somatic mutations BRAF V600E and NRASQ61R with a sensitivity cut-off down to 0.1%, as well as KRAS G12D and KRAS G13D mutations. These mutations have been identified in approximately 50% of thyroid cancers with BRAF V600E present in up to 60% of papillary thyroid cancers.
We have run ThyroiNowTM in hundreds of cases that have proven to help patients in the management of their conditions.
ThyroiNowTM FAQ:
• Detects somatic mutations identified in 50% of thyroid cancers
• Droplet digital PCR technology yields accurate, precise results
• Quick TAT (3-5 day turnaround time)
• Helps patients avoid unnecessary surgery and expense
Ready to Order ThyroiNowTM?
To order, download our requisition, then call us at 855.GOPATH9
1. Med Clin North Am. 2012 March ; 96(2): 329–349. doi:10.1016/j.mcna.2012.02.002